Supernova
Device Description
The Supernova Revascularization Device is a nitinol, self-expanding, laser cut retriever head joined with a delivery wire. Tantalum radiopaque marker coils are wrapped along the retriever head length to aid in visualization under fluoroscopy. The device is loaded into an introducer sheath to reduce its profile and facilitate introduction into the hub of the microcatheter. The delivery wire transfers the force to the retriever head for introducing, tracking, and retrieving the device during treatment.
Intended Use
The Supernova Revascularization Device is intended to restore blood flow and remove thrombus in the cerebral vasculature.
Indications For Use
The Supernova Revascularization Device is indicated for temporary use to restore blood flow in the cerebral vasculature of patients suffering from an acute ischemic stroke.
Intended User
The device should only be used by physicians trained in interventional procedures and who have undergone training in technical aspects of treating acute ischemic stroke. This includes medical specialists including, but not limited to, Interventional Neuroradiologists (INR), Interventional Neurosurgeons, Interventional Neurologists, and others who perform treatment in the neurovasculature via endovascular techniques.
Intended Patient Population
The intended patient population consists of adult persons from the normal population who have been diagnosed with an acute ischemic stroke.
Contents
One (1) Supernova Revascularization Device
Compatibility

Summary Of Characteristics
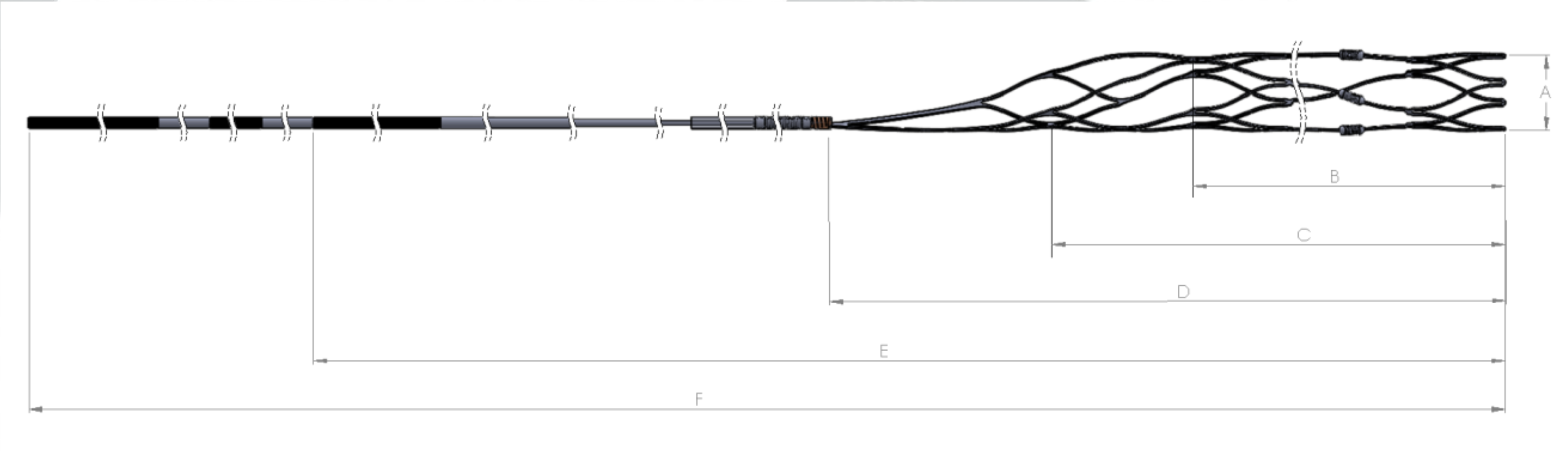

Device selection is based on the sizing recommendation listed in this table and the diameter of the smallest vessel at the thrombus site.
How Supplied
The Supernova Revascularization Device is provided sterile and non-pyrogenic in unopened packaging that is designed to maintain sterility unless the primary product pouch has been opened or damaged. The Supernova Revascularization Device is intended for single use only.
Do not use if package is opened or damaged.
Do not use if labeling is incomplete or illegible.
Storage and Handling: Handle product with care. Store product in a dry and cool place.
Contraindications
There are no known contraindications.
Warnings
- To reduce risk of vessel damage, ensure to appropriately size the Supernova Revascularization Device to the vessel diameter at the intended site of deployment.
- Contents are supplied sterile using an ethylene oxide (EO) process. Do not use product if sterile barrier is damaged or compromised.
- The Supernova Revascularization Device is intended for single use only. Do not reuse, reprocess, or resterilize.
- After use, dispose of the device and packaging per hospital policies.
- The appropriate anti-platelet and anti-coagulation therapy should be administered in accordance with standard medical practice.
- Administer IV t-PA within the currently approved window.
- Do not torque or rotate the Supernova Revascularization Device.
- For vessel safety, do not perform more than 3 recovery attempts in the same vessel using the Supernova Revascularization Device.
- Do not use open or damaged products.
- Do not expose device to solvents.
- Do not advance the microcatheter over the device while it is engaged in clot.
- Do not reposition the device without resheathing within the microcatheter unless thrombectomy is being attempted.
- If withdrawing into the guide catheter is difficult, deflate the balloon guide catheter (if using balloon guide catheter) and then simultaneously withdraw the guide catheter, aspiration catheter or microcatheter, and Supernova Revascularization Device as a unit.
- To reduce risk of kinking/fracture, maintain catheter tip marker over the proximal section of the retriever during manipulation and withdrawal of the retriever.
- Maintain the Supernova Revascularization Device position in vessel when removing or exchanging microcatheter.
- After unsheathing device, position microcatheter or aspiration catheter tip over the proximal section of the Supernova Revascularization Device. Maintain this position during manipulation and withdrawal.
- Use caution when passing the Supernova Revascularization Device through stented arteries.
- Before use, inspect the Supernova Revascularization Device carefully for damage. Do not use a device that shows signs of damage. Damage may prevent device from functioning and may cause complications.
- Do not advance or withdraw the Supernova Revascularization Device against resistance or significant vasospasm. Moving or torquing device against resistance or significant vasospasm may result in damage to vessel or device. Assess cause of resistance using fluoroscopy and, if needed, resheath the device to withdraw.
- If the Supernova Revascularization Device is difficult to withdraw from the vessel, do not torque the Supernova Revascularization Device. Advance the microcatheter or aspiration catheter distally, gently retract the Supernova Revascularization Device back into the catheter and remove the Supernova Revascularization Device and catheter as a unit.
Precautions
- Store in a cool and dry place.
- Do not use after the labeled “Use By” date.
- Exposure to temperatures above 60°C (140°F) may damage device and accessories. Do not autoclave.
- Users should take all necessary precautions to limit X-ray radiation doses to patients and themselves by using sufficient shielding, reducing fluoroscopy times, and modifying X-ray technical factors whenever possible.
- To prevent thrombus formation and contrast media crystal formation, maintain a constant infusion of appropriate flush solution through all catheter lumens.
- Use the Supernova Revascularization Device in conjunction with fluoroscopic visualization and proper anti-coagulation agents.
Expected Clinical Benefits
- Removal of thrombus from occluded cerebral vessels
- Restoration of blood flow in the affected vessels
Risks And Undesirable Side Effects
Possible complications include, but are not limited to, the following:
- Acute Occlusion
- Additional surgery
- Adverse reaction to device materials
- Air embolism
- Cerebral/myocardial ischemia
- Coagulopathy
- Confusion
- Death
- Disability/incapacity
- Distal embolization
- Embolic stroke/myocardial infarction
- False/pseudo aneurysm formation
- Increased procedure time
- Infection
- Inflammatory response
- Intracerebral/intracranial/pericardial hemorrhage
- Neurologic deterioration including stroke
- Pain/headache
- Post-procedure bleeding
- Vascular spasm or vascular occlusion
- Vessel and soft tissue damage
- Vessel thrombosis
Use of device requires fluoroscopy which presents potential risks to physicians and patients associated with x-ray exposure. Possible risks include, but are not limited to, the following:
- Alopecia
- Burns
- Cataracts
- Delayed neoplasia
Adverse Event Reporting
If a device malfunctions or patient complication or injury is experienced or suspected, contact your Gravity Medical Technology representative immediately. Ensure to retain any suspect device, its associated components or accessories and packaging and return to Gravity Medical Technology representative.
Procedure
Device Preparation Delivery Positioning
- Aided by angiographic fluoroscopy, determine the deployment location and its diameter.
- Select Supernova Revascularization Device based on Table 1.
- Flush the Supernova Revascularization Device packaging hoop with saline.
- Prepare and place the balloon guide catheter or guide catheter into internal carotid artery, common carotid artery or subclavian artery per guide catheter instructions for use.
- Connect rotating hemostasis valve to the microcatheter hub. Connect a continuous heparinized saline flush line to the microcatheter.
- With the aid of a guidewire, advance the microcatheter until the end of the microcatheter is positioned distal to the thrombus.
- Remove the guidewire from the microcatheter. Perform a contrast injection through the microcatheter to evaluate the sizing of the branch where the device will be deployed.
- Remove the Supernova Revascularization Device from the dispenser hoop.
- Insert the distal end of the introducer sheath into the microcatheter rotating hemostasis valve, tighten the rotating hemostasis valve, and verify that the fluid exits the proximal end of the introducer sheath.
- Seat the introducer sheath into the microcatheter hub and close the rotating hemostasis valve tightly to secure the introducer sheath in place.
- Advance the Supernova Revascularization Device until the pusher body coil is inserted into the microcatheter. Remove the introducer sheath.
- Advance the Supernova Revascularization Device until the fluorosafe marker enters the hub of the microcatheter.
- Once the fluorosafe marker enters the microcatheter hub, advance the Supernova Revascularization Device under fluoroscopic guidance.
- Advance the Supernova Revascularization Device until the distal tip aligns with the distal tip of the microcatheter.
- Unsheathe the device by withdrawing the microcatheter in the proximal direction and applying gentle forward force to the Supernova Revascularization Device to deploy the retriever head section within the clot. Position the microcatheter tip marker over the proximal section of the Supernova Revascularization Device.
- If resheathing of the Supernova Revascularization Device is necessary, proceed to the following:
- Loosen the RHV around the microcatheter and around the delivery wire. With the aid of fluoroscopic monitoring, hold the delivery wire firmly in its position to prevent the device from moving.
- b. Carefully re-sheath the Supernova Revascularization Device by advancing the microcatheter over the device until the distal markers are retracted back into the microcatheter tip.
Retrieval
- If using an aspiration catheter, position the aspiration catheter just proximal to the microcatheter tip, then remove the microcatheter.
- After deploying the Supernova Revascularization Device, visualize strut expansion and allow sufficient time for clot to integrate into the device (approximately 2 minutes).
- If using a balloon guide catheter, inflate the balloon to occlude the vessel as specified per the device instructions for use.
- If using an aspiration catheter, position the aspiration catheter tip marker over the proximal section of the Supernova Revascularization Device. Tighten the RHV on the microcatheter or aspiration catheter to lock the pusher wire in place. Use a 60 mL syringe to apply aspiration to the aspiration catheter.
- Maintain aspiration while withdrawing the aspiration catheter and Supernova Revascularization Device as a system.
- Slowly withdraw the Supernova Revascularization Device and catheter system to the guide catheter tip while holding aspiration through the guide catheter using a 60 mL syringe.
- Continue aspiration until the Supernova Revascularization Device and catheter system are withdrawn to the guide catheter hub.
- Disconnect the guide catheter rotating hemostasis valve and fully remove the Supernova Revascularization Device and catheter system from the guide catheter.
- Attach a 60 mL syringe to the guide catheter hub and aspirate.
- Deflate the balloon guide catheter balloon.
- Clean the device with saline and inspect the Supernova Revascularization Device for damage. If no damage is present, load the introducer sheath over the proximal end of the delivery wire. The device may be used for up to three (3) retrieval attempts.
- If there is damage, discard the device and use a new Supernova Revascularization Device starting with the device preparation steps.
Summary Of Safety And Clinical Performance
The summary of safety and clinical performance (SSCP) is intended to provide public access to an updated summary of clinical data and other information about the safety and clinical performance of the Supernova Revascularization Device. The SSCP is available in the European database on medical devices (EUDAMED), where it is linked to the Basic UDI-DI.
EUDAMED Website Link:
https://ec.europa.eu/tools/eudamed
Basic UDI-DI for Supernova Revascularization Device:
0850059169SNVA45
Warranty
Gravity Medical Technology warrants that reasonable care was used in the design and manufacture of this product. Gravity Medical Technology has no control over the conditions of use, patient selection or handling of the device after it leaves its possession. Gravity Medical Technology does not warrant either a good effect or against an ill effect following its use. Gravity Medical Technology shall not be directly or indirectly responsible for any incidental or consequential loss, damage or expenses directly or indirectly arising from the use of this product. Gravity Medical Technology’s sole responsibility in the event Gravity Medical Technology determines the product was defective when shipped by Gravity Medical Technology, shall be the replacement of the product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether expressed or implied by operation of law or otherwise, including but not limited to any implied warranties of merchantability or fitness for use.